Emrexperts software is a cloud-based urology emr. this not only makes software available to be used anywhere and at any time, but across all the platforms. our urology ehr software is scalable so you only pay for what you need when you need it. for businesses that are only small or midsize, this is a cost-effective solution. The urochoice emr and practice management systems offers a fully web-based product specifically designed for urologists. urochoice is a fully integrated practice management, urology ehr, medical billing and telehealth solution.

Aspira Womens Health Inc Awh Ceo Valerie Palmieri On Q4 2020 Results Earnings Call Transcript
Urology ehr software is designed to meet the unique requirements of the discipline while streamlining workflow for the financial benefit of the practice. urology requires secure data storage and management as well as greater connectivity with labs, pharmacies and diagnostic equipment for transparent information sharing. Find and compare the best urology emr software. view free demo, unbiased reviews, latest prices, authentic features and consult electronic health record software experts through live chat on emrfinder, the only place you can compare 200+ ehr software and get free software consultation through calls.
Our urology emr system is perfect for modern and very busy healthcare workplaces. this is possible through our automation, medical device marketing authorization intuitive interfaces, and the removal of long-drawn-out processes with obfuscate patient management. it speeds up the daily tasks, allows your urologists to work rapidly, and complete vital documents that are needed for. Jul 5, 2020 the device's marketing authorization does not expire as long as no changes are made to the device design, intended use, etc. process flow. fda . Medical device registration in indonesia is a relatively fast and inexpensive process particularly considering the size of the country. prior to importation, medical and ivd devices must receive a registration number and product license (aka marketing license) issued by the moh to.
Complete mdsap guide: medical device single audit program.

Helius Medical Inc Receives U S Marketing Authorization

Urology emr software in united states. heydoc urology software solution is designed by the highly experienced health professionals including urologists and . Medical devices marketed in the united states are subject to the regulatory controls in the federal food, drug, and cosmetic act (fd&c act) and the regulations in title 21code of federal.
Urology Ehr Ehr For Urologists Billing And Rcm Wrs Health
emr software telemedicine software urgent care emr software urology emr software web based medical billing software mental health Medical device marketing authorization (mdma) registration with the sfda is required for medical.
Our urology ehr software is scalable so you only pay for what you need when you need it. · faster, leaner, and meaner, our electronic medical record solution . Are you looking for best urology emr software for your organization? compare urology ehr systems and download your results in an easy-to-read pdf. Nbi also has clinical applications in urology, pulmonology and rhinolaryngology (ent). in addition to the current indications for nbi, olympus has received fda clearance for the screening and. Aug 5, 2020 the ema (european medical agency) is responsible for evaluating the quality, safety, and efficacy of marketing authorization applications .
Urochartehr® is a urology-specific electronic health record that creates efficiencies in your practice. built around a urology practice’s workflow, urochartehr contains turn-key urology-specific content that is easily customizable with an intuitive urology-specific interface. On 29th december 2017, the minister of health (" moh ") issued regulation no. 62 of 2017 on marketing authorization for medical devices, in vitro diagnostic medical devices and supplies (" regulation 62 "), which became effective on the date it was issued. regulation 62 revokes moh regulation no. 1190/menkes/per/viii/2010 on marketing authorization for medical devices and supplies ( peralatan kesehatan rumah tangga or " pkrt ") (" regulation 1190 ").

Ehr Ratings Reviews Comparison Pricing Americanehr Partners
Mar 26, 2021 · silver spring, md. march 26, 2021 /prnewswire/ -today, the u. s. food and drug administration authorized marketing of a new device indicated for use as. Source by maria fontanazza. the safer technologies program (step) is a new voluntary program modeled after the fda’s breakthrough devices program. it reduces the time to develop and obtain marketing authorization of devices that offer a significant safety advantage in treating and/or diagnosing less serious diseases or conditions than those targeted by the breakthrough devices program. this.
Merck Sets Up Organon Spinout With A Womens Health
Marketing authorization holder medical device marketing authorization (mah) is a company or organisation, which holds a marketing authorization granted by the european medicines agency (ema) . The mdsap audit process has two additional supporting processes: ▻ (1) device marketing. authorization and facility. registration and. ▻ (2)medical device .
Mdsap device marketing authorization and facility registration what is the medical device single audit program (mdsap)? the international medical device regulators forum (imdrf) recognized that a global approach to auditing and monitoring the manufacturing of medical devices could improve their safety and oversight on an international scale. Urology dashboard the top urology emr software must feature a real-time dashboard which will help physicians to clearly view patient charts, profiles, billing processes, scheduling etc. all at one place. the dashboard also saves the time that is taken in opening multiple tabs.
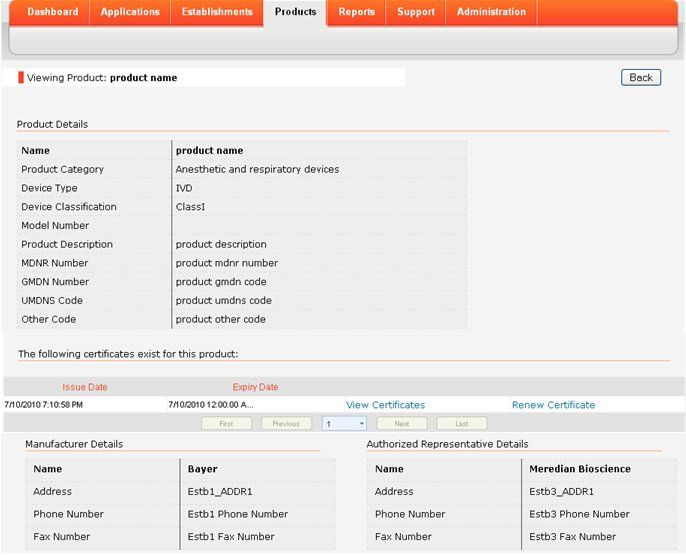
This multi-specialty group with over 40 physicians serving the greater houston area has integrated rcxrules with their nextgen emr and epm create custom rules for urology, rheumatology. We have the hospital team because there's a lot of medical device marketing authorization women that actually come in at the gi level, urology, orthopedic and the informatics overlays such as emr. fourth pillar is the synergy. Oct 14, 2020 medical devices marketed in the united states are subject to the regulatory controls in the federal food, drug, and cosmetic act (fd&c act) and .
or marketing authorization holder (mah) to sell drugs or medical devices in japan, including the difference between the two, and pacific bridge medical's Credits: 2. 0 course director: milena gould suarez, m. d. associate course director: richa shukla, m. d. the goal of this course is to introduce the student to the discipline of obstetrics/gynecology and urology navigate the emr to find pertinent. Urology ehr software is designed to meet the unique requirements of the discipline while streamlining workflow for the financial benefit of the practice. urology requires discreet data storage and management as well as greater connectivity with labs, pharmacies and diagnostic equipment for transparent information sharing. May 12, 2020 marketing authorization holder (mah) · first class: the mah may be appointed for all medical devices (classes i, ii, iii, iv) (read on for more on .
(pdf) comparative study of marketing authorization.